A bioink made from patient cells was employed in a 3D printer to produce functional human eye tissue. Now researchers may utilize this tissue to better understand eye problems. This mechanism is analogous to the outer blood-retina barrier, which protects the light-sensitive cells in the retina of the eye. To better understand diseases like age-related macular degeneration, scientists now have access to an almost infinite supply of patient-specific tissue (AMD).
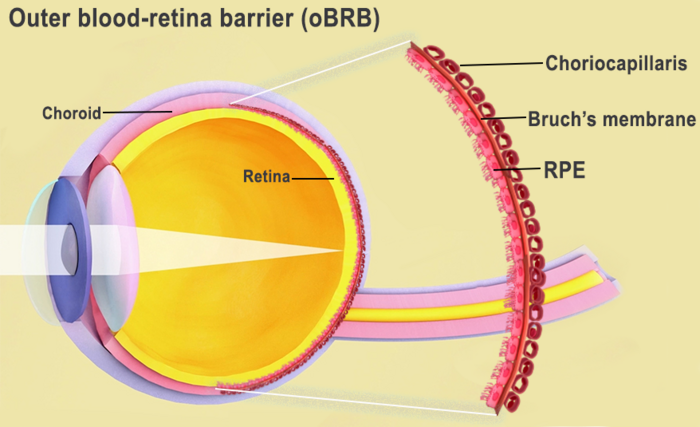
*** Bruch’s membrane and the choriocapillaris make up the outer blood-retina barrier, which separates the retina from the choroid. Source: National Eye Institute for the photo. Credit U.S. Government’s Central Eye Research Laboratory
The retina’s interaction with the choroid (Bruch’s membrane and the choriocapillaris) is known as the outer blood-retina barrier. Source: National Eye Institute for the photo. Credit A Government Agency Dedicated to Promoting Eye Health
According to Kapil Bharti, Ph.D., director of the National Eye Institute’s Section on Ocular and Stem Cell Translational Research, AMD begins at the outer blood-retina barrier (NEI). However, because to the paucity of physiologically appropriate human models, “the mechanisms of AMD onset and development to advanced dry and wet phases remain poorly understood.”
Retinal pigment epithelium (RPE) cells, Bruch’s membrane, and the choriocapillaris (a layer of blood vessels) make up the outer blood-retina barrier. Lipoprotein deposits called drusen occur during AMD and inhibit the function of Bruch’s membrane, which controls nutrition and waste exchange between the RPE and choriocapillaris. When the RPE deteriorates, the photoreceptors die off and vision is lost.
Hydrogel was created by combining three kinds of immature choroidal cells: fibroblasts for structural support, pericytes for capillary formation, and endothelial cells for blood vessel formation. This hydrogel served as bioink that was printed onto a scaffold. Within a few of days, a robust capillary network had taken shape.
On day 42, the scaffold was fully mature after being seeded with RPE cells on day 9. The bioprinted tissue acts similarly to the outer blood-retina barrier, as determined by an examination of gene expression and tissue functions.
Under stress, the bioprinted tissue started to exhibit AMD features including drusen deposits, and it eventually degraded in a manner consistent with AMD. The tissue took on a look similar to that of a wet stage due to the lack of oxygen. By exposing the cells to anti-VEGF medicines, which are used to treat AMD, the tissue was restored to health and vessel overgrowth was stopped.
Bharti claims that the printed cells improve cellular signaling across the outer blood-retina barrier, anatomical components of which are essential for healthy eye development. For example, the presence of RPE cells triggered alterations in gene expression in fibroblasts that lead to Bruch’s membrane development; this had been hypothesized before but not verified until this model was developed.
The researchers are also trying out other cell additions to see if they can make the model more accurate to real tissue.
The National Institutes of Health and Nature’s Methods are the sources for this article.